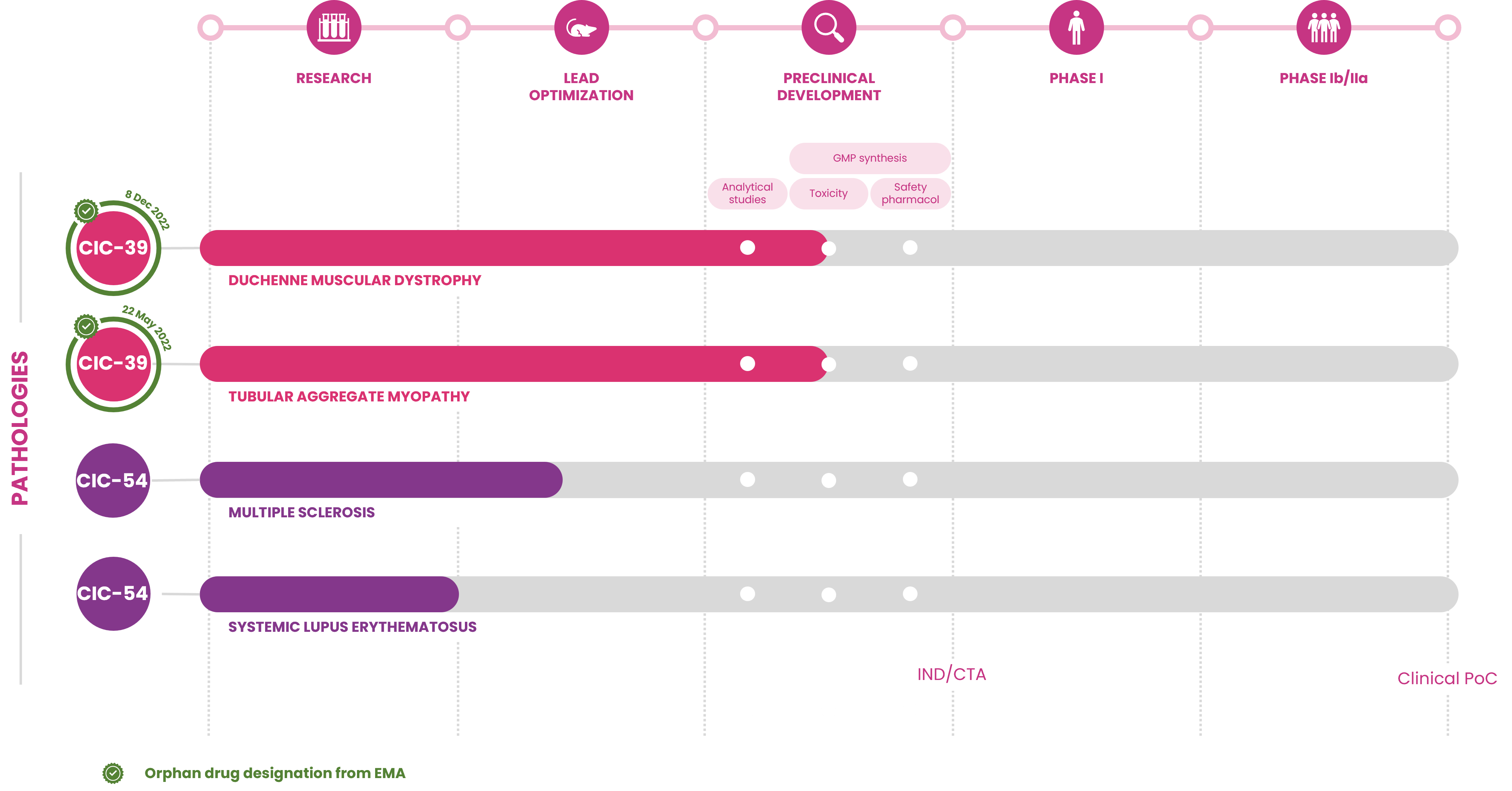
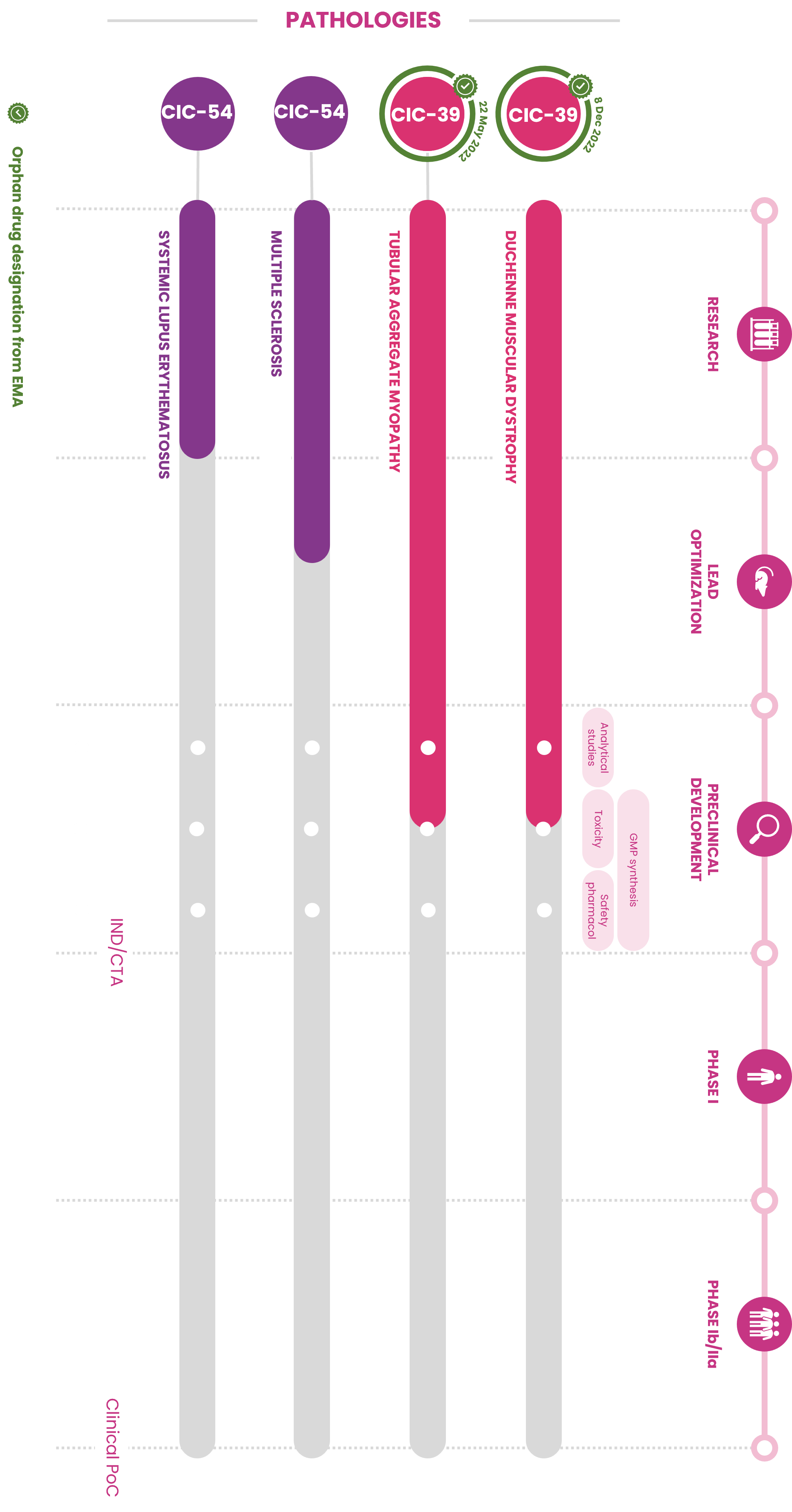
Our technology

Good safety & pharmacokinetic profile
Test passed:
- genotoxicity: in vitro micronucleus test in human lymphocytes
- mutagenicity: Ames Test in Salmonella Typhimurium and Escherichia coli
- oral bioavailability: comparative pharmacokinetic analysis in vivo versus gavage administration
- cardiac safety: hERG channel test
* according to the OECD guidelines and Good-Laboratory Practice (GLP) criteria

IP protection

Easy manufacturing
- parallel preparation of a large number of molecules;
- straightforward exploration of the chemical space during the early drug discovery phase;
- easy chemical refinement of hit compounds during the hit-to-lead process in order to meet the pharmacokinetic and pharmacodynamic requirements.